As you are heading towards
the end of january, you might be in need of many important tips for your
revision process...
Here are few such in the topic Real Gases.
Gases that don’t obey  equation are known as “Real
gas”.
equation are known as “Real
gas”.
 equation are known as “Real
gas”.
equation are known as “Real
gas”. .
.
If Z < 1, there exists net attraction between gas
molecules
If Z > 1, there exists net repulsion between the
gas molecules.
Vander waal’s equation: The gas which obeys Boyle’s law, charle’s law for all
values of temperatures and pressure is “Ideal gas”. But no gas is ideal or
perfect in nature. So, for real gas this theory was given.
Where a and b are characteristics constants of a real
gas.
Volume
correction:
Vander
waal’s assumed that molecules of real gas are rigid spherical particles which
posses a definite volume. Thus, the volume of real gas i.e., volume available
for compression or movement is actual volume minus volume occupied by gas
molecule.
Note:
 i.e., at very high temperature and very low pressure, a
non-ideal gas becomes an ideal gas.
i.e., at very high temperature and very low pressure, a
non-ideal gas becomes an ideal gas. not V.
not V.
Corrected volume = V – b for 1 mole of a
gas for n moles, corrected volume =  .
.
 .
.
Where,
r is the radius of gas molecule
N
= Avogadro number
Pressure
correction:
A
molecule in the interior of the gas is attracted by other molecules on all
sides. A gas molecule which is just going to strike the wall of the vessel
experiences an inward pull due to attractive forces.
Hence,
it strikes the wall with less momentum and the observed pressure will be
less than the ideal pressure.
P'
is the pressure correction
 .
.
This
equation is called real gas equation depending on a and b the gas behavior
changes.
Units
of a and b:-
1. Pressure correction
 .
.
Units of a :-
S.I. units of a  .
.
 .
.
Unit of b:-
Volume correction .
.
 .
.
Unit of b .
.
 .
.






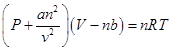



No comments:
Post a Comment