Defects in Non-Stoichiometric Compounds: The compounds in which the ratio between numbers of anions to number of anions is not equal to ratio indicated by the formula.
- Metal Excess Defect
- Metal Deficiency Defect.
i. Anion Vacancy:
⇒ This free electron center is called F-centre.
⇒ Due to this only these compounds exhibits colour.

ii. Interstitial cation:
⇒ In this one of the cation will present at the interstitial position and which
is stabilized by the electron at interstitial position.
⇒ In this the number of cations are not equal to the number of anions.

b. Metal Deficiency Effect:
i. Cation Vacancy: One cationic space is empty instead of that a positive cation will present in order to maintain the electrical neutrality.
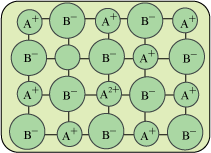
ii. Interstitial Anion: In this defect the anion occupies interstitial position and one of the cation will be in its +2 oxidation state.



No comments:
Post a Comment