No: of atoms per unit cell:
Position of Atoms
in the Unit Cell
|
Contribution
towards One Unit Cell
|
Corners
|
⅛
|
Edges
|
¼
|
Face centered
|
½
|
Body Centered
|
1
|
Primitive cell:


8 atoms at corners
So no. of atoms per cell = 8 x ⅛ = 1
Body centered:
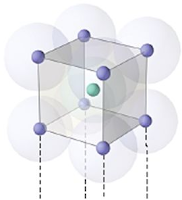

Total atoms at corners = 8
1 atom at centre
So total no: of atoms per unit cell =
(8 x ⅛) + 1 = 2
Face centered unit cell:
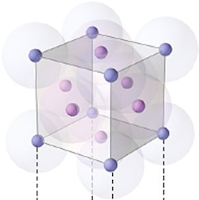

No: of atoms at corner = 8
No: of atoms at face = 6
So total no: of atoms per unit cell =
(8 x ⅛) + (6 x ½) = 4
Crystal Radius:
Simple Cube:


a = 2r
r = a/2
Face Centered:

b = 4r
b² = a² + a²
16r² = 2a²
a = √8 r
a = 2√2 r
r = a/2√2
Body Centered:

b² = a² + a²
c² = a² + b²
c² = 3a²
c = √3 a
a = 4r/√3
r = √3a/4
Coordination number of unit cell: The number of nearest neighbors that a
particle has a crystal lattice is called Coordination number:
1) For simple coordination number = 6
2) For Face centered cubic lattice = 12
3) For Body centered cubic lattice = 8

No comments:
Post a Comment