⇒
Phosphorus
forms a large number of oxoacids.
⇒
All these
acids are based on tetrahedral four coordinated phosphorus atom containing
at least one P = O unit and one P - OH group. Condensed systems are formed by P
- O - P linkage or P - P linkage.
⇒
The basicity
depends on the H atoms bonded to O in the form of - OH.
Some of the common oxoacids of phosphorus are
as follows:
Name
|
Formula
|
Oxidation
state of P
|
basicity
|
structure
|
preparation
|
Hypophosphorus
acid
|
H3PO2
|
+1
|
1
|
 |
white P4
+ alkali
|
Phosphorus
acid
|
H3PO3
|
+3
|
2
|
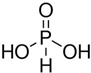 |
P2O3
+ 3H2O → 2H3PO3
|
Hypophosphoric
acid
|
H4P2O6
|
+4
|
4
|
 |
red P4
+ alkali
|
Orthophosphoric
acid
|
H3PO4
|
+5
|
3
|
 |
P(red) + 5HNO3 → H2O + H3PO4 + 5NO2
or
P4O10
+ 6H2O → 4H3PO4
|
Diphosphoric
acid (Pyrophosphoric acid)
|
H4P2O7
|
+5
|
4
|
 |
heating
phosphoric acid
2H3PO4
→ H4P2O7 + H2O
|
Metaphosphoric
acid
|
HPO3
|
+5
|
1
|
 |
heating
orthophosphoric acid to about 850 K
H3PO4
→HPO3 + H2O
|
Peroxophosphoric
acid
|
H3PO5
|
+7
|
3
|
 |
P2O5
+ 2H2O2 + H2O → 2H3PO5
|
Oxides
of phosphorous:
1. Phosphorus pentoxide:
⇒ It
is a chemical compound with molecular formula P4O10 (with its common name derived from its empirical formula, P2O5).
⇒ This white crystalline solid
is the anhydride of phosphoric
acid. It is a powerful desiccant and dehydrating
agent.
⇒ P4O10 is
prepared by burning elemental phosphorus with sufficient supply of
air:
P4 + 5 O2 →
P4O10


2. Phosphorus trioxide:
⇒ It
is the chemical compound with the molecular formula P4O6.
Although it should properly be named tetraphosphorus hexoxide, the name
phosphorus trioxide preceded the knowledge of the compound's molecular
structure, and its usage continues today.
⇒ It is a colorless solid. It
is formally the anhydride of phosphorous
acid, H3PO3, but cannot be obtained by the dehydration of
the acid. It is a white, waxy, crystalline and highly toxic solid.
⇒ It is obtained by
the combustion of phosphorus in a limited supply of air at low temperature.
P4(s) + 3 O2 (g)
→ P4O6(s)



Thank you for sharing that great information.
ReplyDeleteAlso, find best Phosphoric Acid Suppliers Who offers the best quality Phosphoric Acid in bulk.