Crystal lattice is the depiction of three dimensional
arrangements of constituent particles (atoms, molecules, ions) of crystalline
solids as points.

Characteristics of Crystal Lattice:
- Each constituent particle is represented by one point in a crystal lattice.
- These points are known as lattice point or lattice site.
- Lattice points in a crystal lattice are joined together by straight lines.
- By joining the lattice points with straight lines the geometry of the crystal lattice is formed.

Parameters of a Unit Cell:
- A unit cell is characterized by six parameters. These parameters are three edges (a, b and c) and angles between them (α, β and γ).
- Dimensions along the edges of a unit cell is represented by a, b and c.
- Edges of unit cell may or may not be mutually perpendicular.
- The angle between b and c is represented by α, between a and c by β and between a and b by γ.
1. Primitive Unite Cells: When particles in unit cell are
present only at the corners, it is called the primitive unit cell.

2. Centered Unit Cells: When particles are present at
other positions in addition to those at corners in a unit cell, it is called a Centered
Unit Cell. There are three types of Centered Unit
Cell.
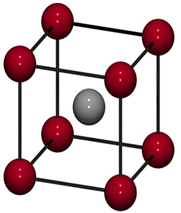
(a) Body Centered Unit Cells: If one constituent particle lies
at the centre of the body of a unit cell in addition to the particles lying at
the corners, it is called Body-Centered Unit Cell.
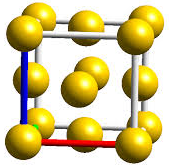
(b) Face Centered Unit Cells: If one constituent particle lies
at the centre of each face besides the particles lying at the corner, it is
known as Face-Centered Unit Cells.
(c) End Centered Unit Cell: If one constituent particle lies
at the centre of any two opposite faces besides the particles lying at the
corners, it is known as End-Centered Unit Cell. It is also known as base-centered
unit cell.



No comments:
Post a Comment