Qualitative chemical
analysis deals with the
identification of elements or grouping of elements present in a sample.
Detection of Carbon and Hydrogen: Carbon and hydrogen are detected by heating the compound with copper
(II) oxide. Carbon present in the compound is oxidised to carbon dioxide
(tested with lime-water, which develops turbidity)
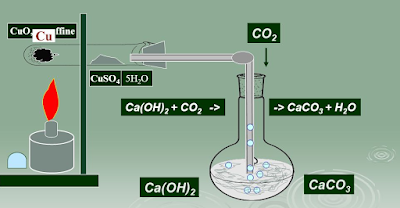

C + 2CuO → 2Cu + CO₂
CO₂ + Ca (OH)₂ → CaCO₃↑ + H
Hydrogen to water (tested
with anhydrous copper sulphate, which turns blue).
2H + CuO → Cu + H₂O
5H₂O + CuSO₄ (White) → CuSO₄.5H₂O (Blue)
Sodium Fusion Test: In order to detect nitrogen, sulphur and halogen in organic compounds, it is necessary to convert them into ionisable inorganic substances so that ionic tests of to fure the organic compounds with metallic sodium (Lassaigne’s test). In this way sodium cyanide, sodium sulphide and sodium halides, which are readily elements are present.
1. Test for
Nitrogen: The sodium fusion extract is
boiled with iron (II) to form sodium hexacyanoferrate (II).
6CN⁻ + Fe²⁺ → [Fe (CN)₆]⁴⁻
On heating with concentrated sulphuric
acid some iron (II) ions are oxidised to iron (III) ions which react with sodium
hexacyanoferrate (II) to produce iron (III) hexacyanoferrate (II)
(ferriferrocyanide) which is Prussian blue in colour.
3[Fe (CN)6]⁴⁻ + 4Fe³⁺ → Fe₄ [Fe (CN)₆]₃.xH₂O
Prussian blue
2. Test for
Sulphur:
a) The sodium fusion extract is
acidified with acetic acid and lead acetate is added to it. A black precipitate
of lead sulphide indicates the presence of sulphur.
S²⁻ + Pb²⁺ → PbS (Black)
b) On treating sodium fusion
extract with sodium nitroprusside, appearance of a violet colour further
indicates the presence of sulphur.
S²⁻ + [Fe (CN)₅ NO]²⁻ → [Fe (CN)₅ NOS]⁴⁻ (Violet)
c) In case, nitrogen and sulphur both are present in an organic
compound, sodium thiocyanate is formed. It gives blood red colour.
Na + C + N + S à NaSCN
Fe³⁺ +SCN⁻ → [Fe (SCN)]²⁺
3. Test for
Halogens: The sodium fusion extract is
acidified with nitric acid and then treated with silver nitrate.
X⁻ + Ag⁺ → AgX {X represents a halogen – Cl, Br or I}
Presence of chlorine: A
white precipitate, soluble in ammonium hydroxide.
Presence of bromine: A
yellowish precipitate, sparingly soluble in ammonium hydroxide.
Presence of iodine: A yellow
precipitate, insoluble in ammonium hydroxide.
If nitrogen or sulphur is also present in the compound, the
sodium fusion extract is first boiled with concentrated nitric acid to decompose
cyanide or sulphide of sodium formed during Lassaigne’s test. These ions would
otherwise interfere with silver nitrate test for halogens.
4. Test for
Phosphorus: The compound is heated with an oxidizing
agent (sodium peroxide). The phosphorus present in the compound is oxidised to phosphate.
The solution is boiled with
nitric acid
Na₃PO₄ + 3HNO₃ → H₃PO₄+3NaNO₃
It is then treated with
ammonium molybdate. A yellow colouration or precipitate indicates the presence
of phosphorus.
H₃PO₄ + 12(NH₄)₂ MoO₄ + 21HNO₃ → (NH₄)₃PO₄.12 MoO₃ + 21NH₄NO₃ + 12H₂O
Ammonium Molybdate Ammonium Phosphomolybdate

No comments:
Post a Comment